Terrie Cowley, Co-Founder and President of The TMJ Association, often remarks that patients tell her that the pain they feel in their jaws is worse than pain elsewhere in the body. People with migraines, cluster headaches or trigeminal neuralgia would agree. Now you might guess that this is because the face is more generously supplied with nerve endings than other parts of the body (true) and the ratio of nerves to muscles is high. And that is why you can make such exquisitely fine facial movements and expressions. But a more exciting and interesting finding has emerged from experiments by researchers at Duke University in Durham, North Carolina, and reported recently in Nature Neuroscience.[1] The team discovered a direct route from the neurons that sense pain in the face or head to neurons in the brain that connect to key emotional centers, areas that evoke the suffering associated with pain and the fear, anxiety and depression that so often go along with it.
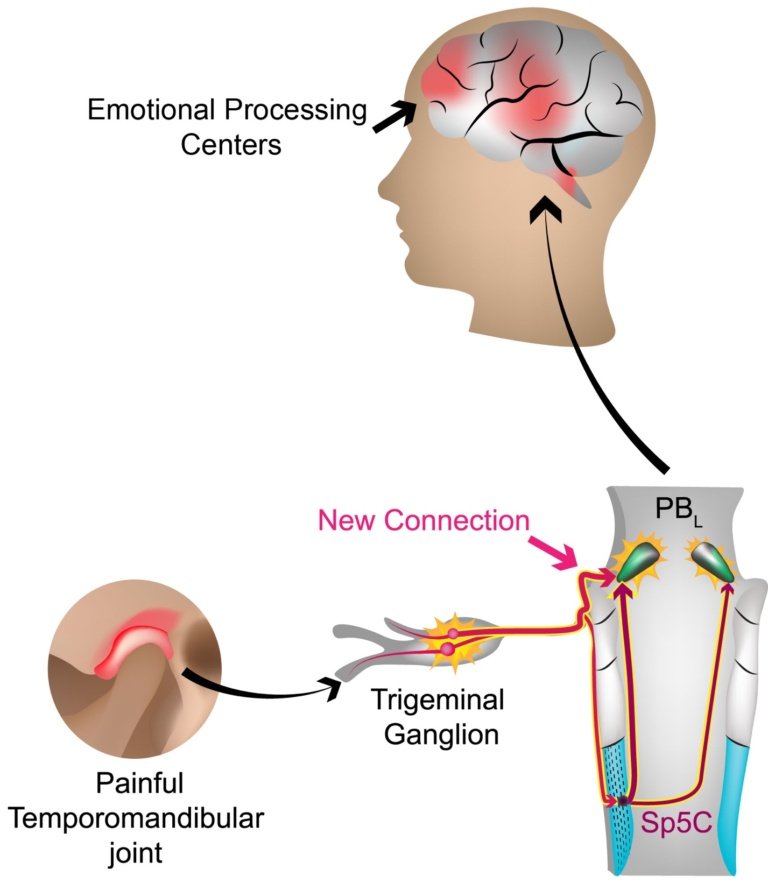
You need some neuro-anatomy to understand this.In the head, the neurons that send sensory fibers out to facial and head areas have their cell bodies in the trigeminal ganglion, a cluster of neurons that lies just outside of the brain, one on each side of the head.
The ganglion cells include those sensitive to touch, pressure, heat and cold as well as pain, whose fibers contact the meningeal membranes inside the skull,relevant for all headaches,the cornea of the eye, the teeth, tongue, sinuses, lips,and facial skin. From the ganglion the pain-sensing nerve cells, nociceptors, transmit signals along fibers at their other end (their axons) that connect with the brain. Some project to areas where they signal the painful TMJ traveled to the Sp5C where it was synaptically relayed to the PBL. The hitherto unknown direct plugin connect directly to the PBL is then relayed to emotional processing centers in the brain location of your pain and how intense it is.
Some find their way to cells in the parabrachial (PB) nucleus (clusters of neurons in the brain are called nuclei). There are two parabrachial nuclei that hug either side of a portion of the midbrain, apart of the brain under the cortex and over the spinal cord. The startling finding of the Duke researchers was that there is a direct pathway for facial/head pain signals from the trigeminal ganglion to the lateral part of the parabrachialnucleus and from there to multiple sites in the brain associated with feelings and emotion. The investigators describe this as a one-synapse (“monosynaptic”)pathway-from ganglion cell to lateral PB with no other relay station in between. Of note,this new monosynaptic projection appears to be an exclusive pathway for trigeminally-mediated pain only.
In contrast, pain from the body does not have an express route to feelings but relays to the PB via a way-station in between. Indeed, trigeminal pain neurons were traditionally thought to use an indirect route to the PB through a nucleus in the brain called the spinal trigeminal nucleus caudalis. So what the Duke team discovered is a second streamlined pathway. That in itself offers hope for new ways to treat refractory facial pain because past efforts have only looked at ways to intervene in the traditional pathway. In addition, the investigators demonstrated that the facial/head pain signals to the PB are more extensive than for an equally intense body pain, and also contact PB nuclei on both sides of the brain, even though the pain may have originated on only one side.
To demonstrate these properties, new sophisticated technologies were used, including one invented by Fan Wang, senior author of the paper and chief of the laboratory where the experiments were conducted. A major complication the team faced is that the PB nuclei are made up of many different kinds of neurons receiving a range of sensory inputs, including taste stimuli. They also contain neurons involved in regulating hunger and thirst. Moreover, PB neurons, including nociceptors, use a variety of neuro-transmitters in their operations. Other relevant molecules in nociception include TRPV1,a receptor for the hot pepper ingredient capsaicin, and CGRP, calcitonin gene related peptide, which has an established role in migraine headaches and in sustaining neuro-inflammation.
So the first step for the investigators was to determine which parts of the PB might be rich in nociceptive neurons and the neurotransmitters they used. In experiments with mice, the investigators injected equal amounts of a painful chemical irritant such as formalin into either the whisker pads or the hind paws of the mice. This would stimulate nociceptors in the trigeminal ganglion for the whisker pad or a comparable ganglion adjacent to the spinal cord for the hind paw. From there some pain signals would relay to the PB nuclei. To trace which PB neurons were responsive to pain, the researchers made use of a genetic change in affected nerve cells. It turns out that a sensorystimulus triggers the production of an “immediate-early gene” in the nerve cell, generating a protein of a family called Fos, enabling it to be used as a marker for activated neurons. The investigators could inject formalin unilaterally in the mice, wait for the production of Fos, then extract PB brain tissue and stain it to reveal Fos. In this way they located the lateral PB, especially an external sub nucleus, PB-el, as the locusof PB nociceptive neurons. The same staining method demonstrated that pain neurons excited by signals from the whisker pad were more numerous than those from the hind paw and were found in both PB nuclei rather than predominantly on one side.
Now the question was the source of the face pain inputs to the PB. Did they stem from the traditional indirect route through the trigeminal spinal nucleus caudalus or was there a direct trigeminal ganglion to PB route? To do the back tracing of inputs the Duke team
employed a ground-breaking new technology developed by Dr. Wang called CANE, for Capturing Activated Neuronal Ensembles. The method was used for selectively labeling and remote-controlling a population of pain-activated PB cells. The task to establish the pre-synaptic source for the inputs to the PB-el was more complicated. It required injections of different viral-protein combinations into the PB after formalin injections to mouse whisker pads. These injections were followed two weeks later by yet another combination injection into the same part of the PB. The clincher here was finding that the second injection labelled cells in both the PB-el AND the trigeminal ganglion proving that the ganglion cell was the source of a direct input to the PB nucleus.
Additional experiments established that PB-el neurons project widely to emotional processing centers in the brain such as the amygdala, known to play an important role in generating fear and anxiety. In turn, these same emotional areas also project back into the PB-el in a loop. Finally, the Duke researchers employed yet another sophisticated technique, optogenetics, to investigate pain-related behaviors in mice. They implanted optic fibers in a selected set of trigeminal pain neurons whose axonsterminate in the PB-el. When light of the appropriate wave length is shined on the fibers it activates the nerve cells. The team then observed the behavior of mice exposed to a two-chamber spatial arrangement. Without optic stimulation the mice freely explore the space and may choose one of the two chambers as preferred. When the pain stimulation was turned on while the mice were in the preferred chamber, however, they immediately ran to the opposite chamber often vocalizing with typical stress calls.Other experiments showed that silencing optogenetic stimulation could reverse the aversive behavior and had additional analgesic effects in other pain behavioral tests.In sum, the team interprets these experiments as further evidence of the significance of the direct trigeminal PB connection in pain perception and behavior.
The Future. As noted earlier, the establishment of a second pathway governing face/head pain in relation to emotions and feelings offers new approaches to treatment, perhaps targeting molecules such as TRPV1 or CGRP. In addition, ways to moderate or even selectively sever connections established by the new monosynaptic pathway may provide relief to patients where previous surgery, for trigeminal neuralgia, for example, has been ineffective.

About the team. It is noteworthy that the paper reporting the discovery of the new pathway was based on experiments in Dr. Wang’s laboratory executed as part of the PhD thesis of the first author Dr Erica Rodriguez. She herself is no stranger to pain as a result of sports-related herniated discs and surgery. Dr. Wolfgang Liedtke who also sees patients with “refractory” trigeminal pain disorders in his two clinics at Duke University, and Dr. Yong Chen from his laboratory, also contributed important experiments to the paper. Previously, Chen, Wang and Liedtke described a new method to measure TMJ pain equivalents in laboratory mice. Assessment of effects of TMJ injury and TMJ pain on the newly established trigeminal pain circuits will be an appealing logical next step for the Duke team.
[1]A craniofacial-specific monosynaptic circuit enables heightened affective pain. Rodriguez E, Sakurai K, Xu J,Chen Y, Toda K, Zhao S, Han BX, Ryu D, YinH, Liedtke W, Wang F. Nat Neurosci. 2017 Dec;20(12):1734-1743. doi:10.1038/s41593-017-0012-1. Epub 2017 Nov 13.
Additional References:
Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. ChenY, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C,Gereau RW 4th, Taylor AB,WangF, Guilak F,LiedtkeW. Pain. 2013 Aug;154(8):1295-304. doi: 10.1016/j.pain.2013.04.004. Epub 2013 Apr 6.The referenced work was supported by NIH grants F31 DE025197-03 (EricaRodriguez), K12DE022793 (Yong Chen), DE018549 (Wolfgang Liedtke), DE019440and DP1MH103908 (Fan Wang).
